Our laboratory investigates the mechanisms whereby redox modifications of cysteine residues regulate protein and cell function. We focus on immune cells and cancer cells. Current projects in the lab use cutting-edge proteomic and biochemical tools to explore the roles of cysteine modifications, mainly S-nitrosylation and S-sulfhydration, in cellular communication, inflammation, and cancer.
- The free radical, nitric oxide (NO) is involved in numerous cellular and organismal processes, including cell division, migration, differentiation and death, and vascular and immune function. NO is a central mediator of inflammation, and thereby might play important roles in diseases associated with chronic inflammation, including cancer. NO influences these diverse processes largely through targeted modification of cysteine residues in proteins, generating S-nitrosylated proteins.
- A large body of evidence has established that protein cysteine nitrosylation (S-nitrosylation) plays an important role in a wide range of processes, particularly in mammalian cells. Reversible S-nitrosylation of specific cysteine residues represents a principal mechanism by which NO signals. Much like protein phosphorylation, protein S-nitrosylation influences protein activity, protein-protein interactions and protein location.
- Despite recent progress, the involvement of protein nitrosylation and denitrosylation in inflammatory diseases and cancer is presently not clear. Research in our lab aims to address this major question. We apply novel proteomic approaches to identify proteins regulated by reversible S-nitrosylation. Our research may lead to better understanding of mechanisms that control immunity, inflammation, and tumorigenesis, and eventually will point to novel targets for therapeutic intervention.
- Similar to NO, hydrogen sulfide (H2S) acts as a signaling molecule in diverse cellular processes. H2S-mediated signaling largely occurs via modification of cysteine residues in proteins, a process called S-sulfhydration (or S-persulfidation). We are currently investigating how S-sulfhydration can modulate cellular processes such as immune cell activation and cancer cell apoptosis.
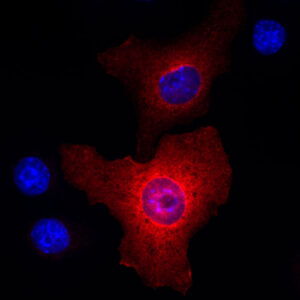
Inducible nitric oxide synthase (iNOS) expression during macrophage activation.
Elevated expression of iNOS plays a central role in the pathophysiology of many inflammatory disorders.
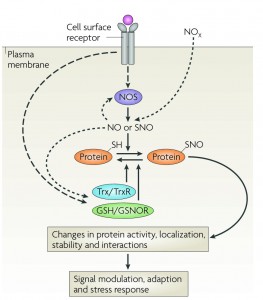
Cellular regulation by reversible cysteine nitrosylation.
Dynamic, stimulus-coupled protein S-nitrosylation and denitrosylation, largely mediated by NOS, GSNO reductase and thioredoxin, influence protein activity and downstream signaling.
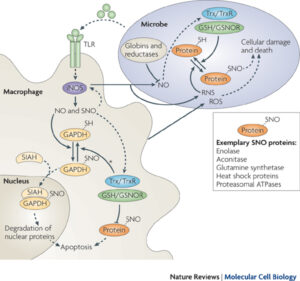
Reversible nitrosylation in immunity
The balance between nitrosylation and denitrosylation affects host-microbe interaction and innate immunity.
